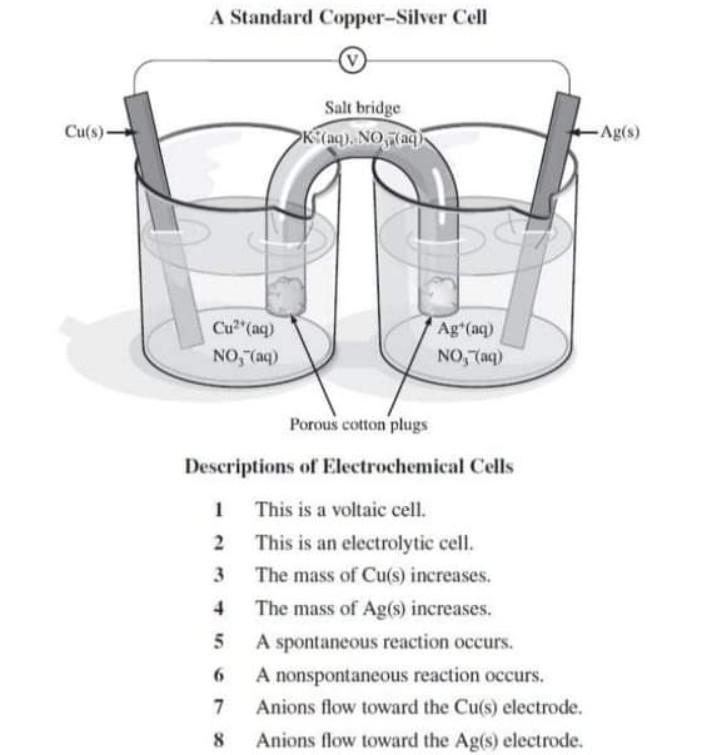
A copper - silver cell is set up. The copper ion concentrations is 0.10 M. The concentration of... - YouTube
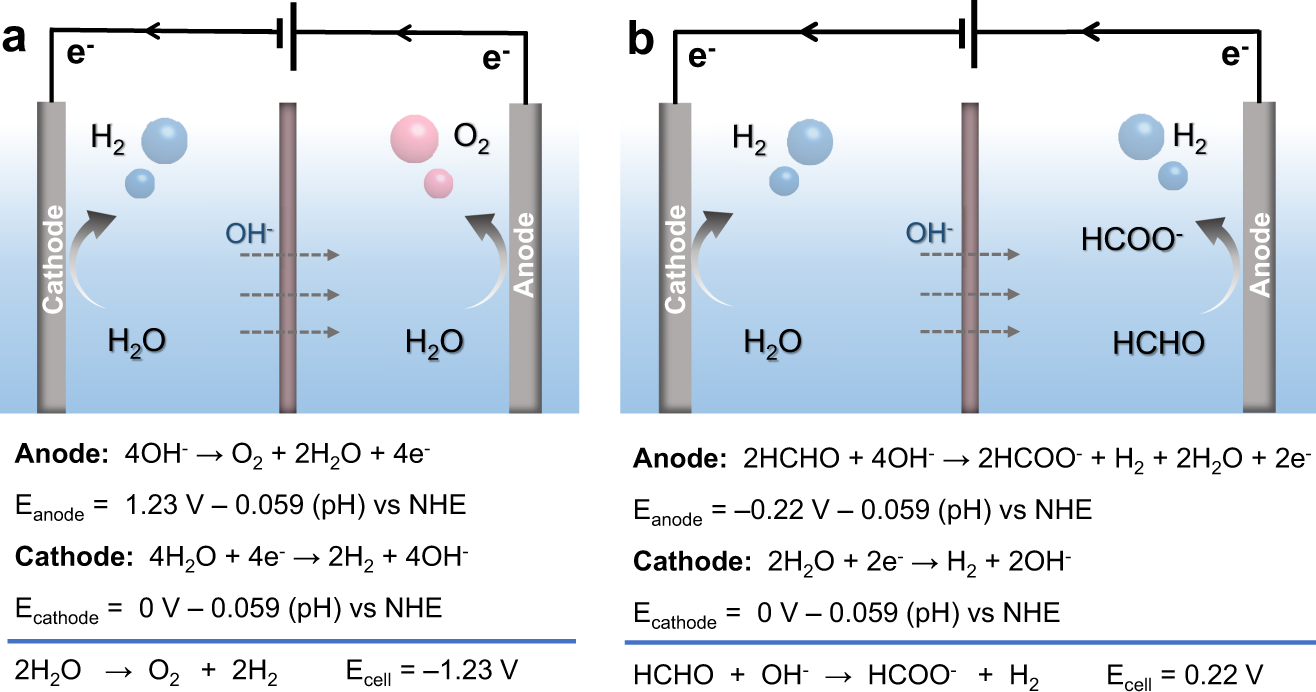
Dual hydrogen production from electrocatalytic water reduction coupled with formaldehyde oxidation via a copper-silver electrocatalyst | Nature Communications
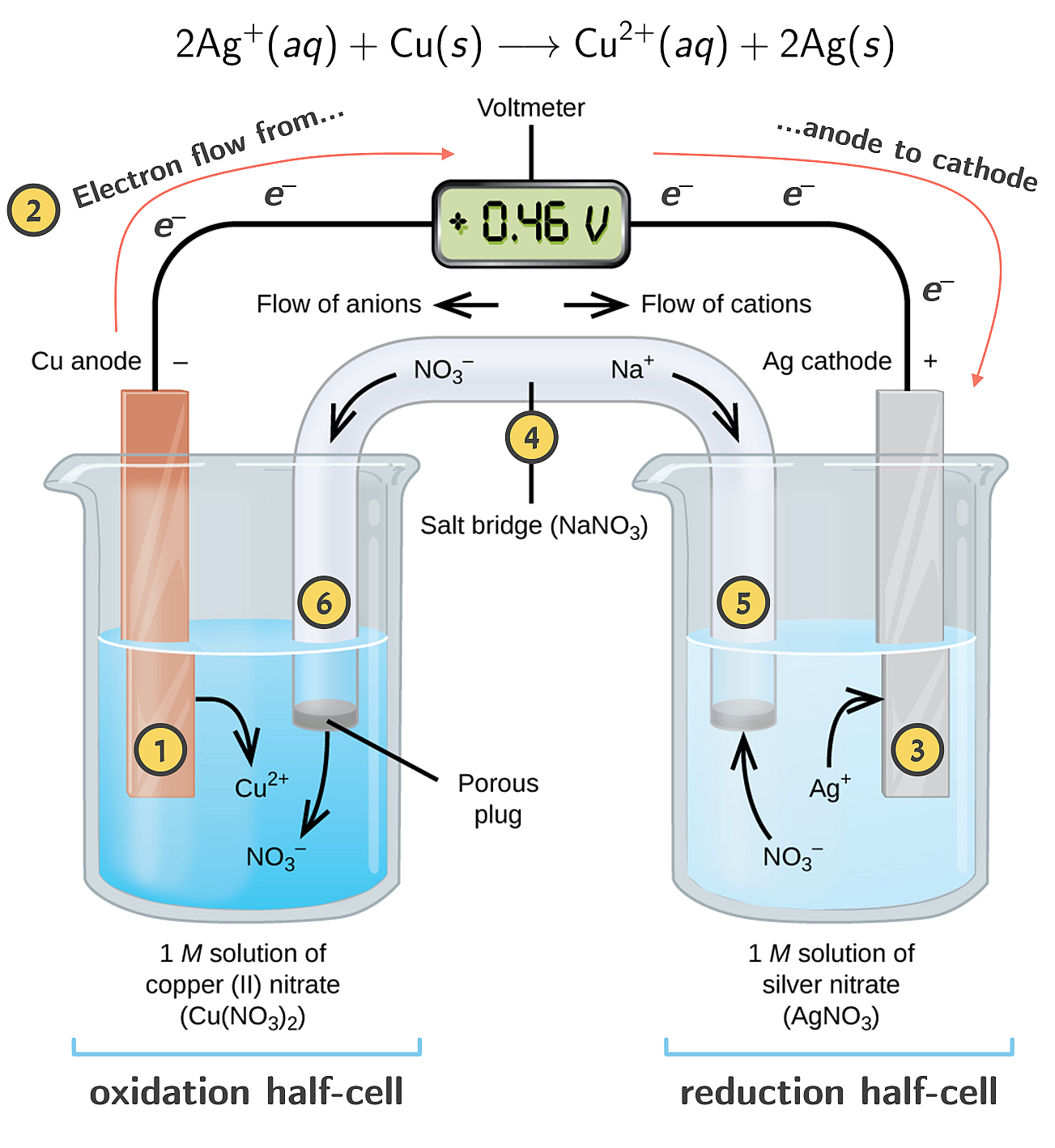
A copper-silver cell is set up. The copper ion concentration is 0.10 M. The Ecell = 0.422 V. Determine the concentration of [Ag^+] in the cell if - Sarthaks eConnect | Largest Online Education Community

HULUSUL PURCULUI 13.A Copper-silver cell is up. The copper ion concentration in it is 0.10M.The concentration of silver is not known. The cell potential measured is 0.422V.Determine the concentration of silver ion
A copper_ silver cell is set up. The copper ion concentration is 0.10 M. The concentration of silver ion is not know.the cell potential when measured was 0.422V determine the concentration of

A copper - silver cell is set up. The copper ion concentrations is 0.10 M. The concentration of... - YouTube

Solve this: 8 A Copper-Silver cell is set up The copper ion - Chemistry - Electrochemistry - 12485096 | Meritnation.com

Following cell is set up between copper and silver electrodes Cu//Cu^(2+)(aq)||Ag^(+)//Ag. If tw... - YouTube

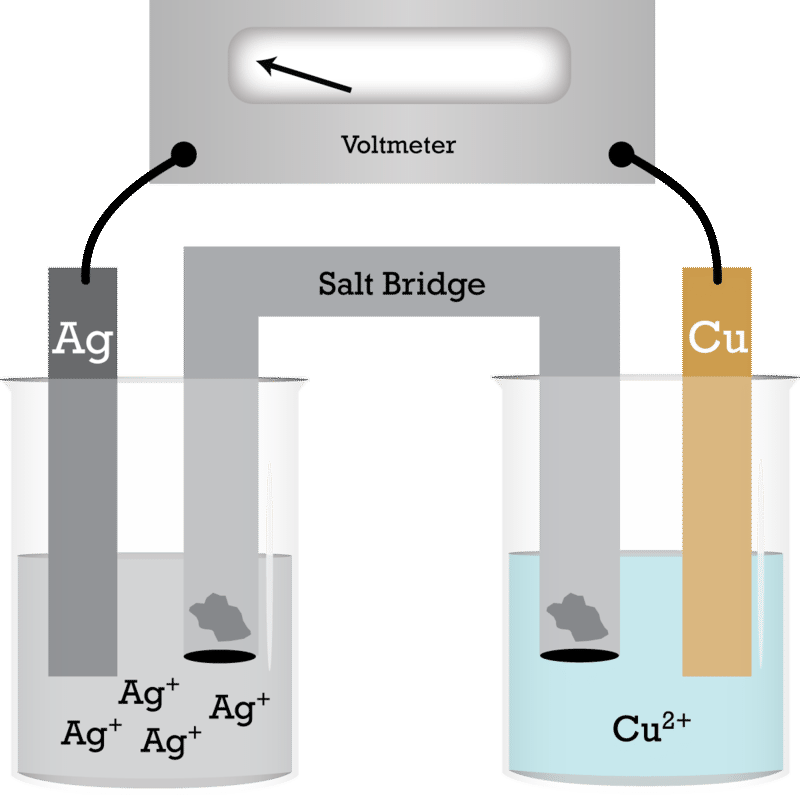
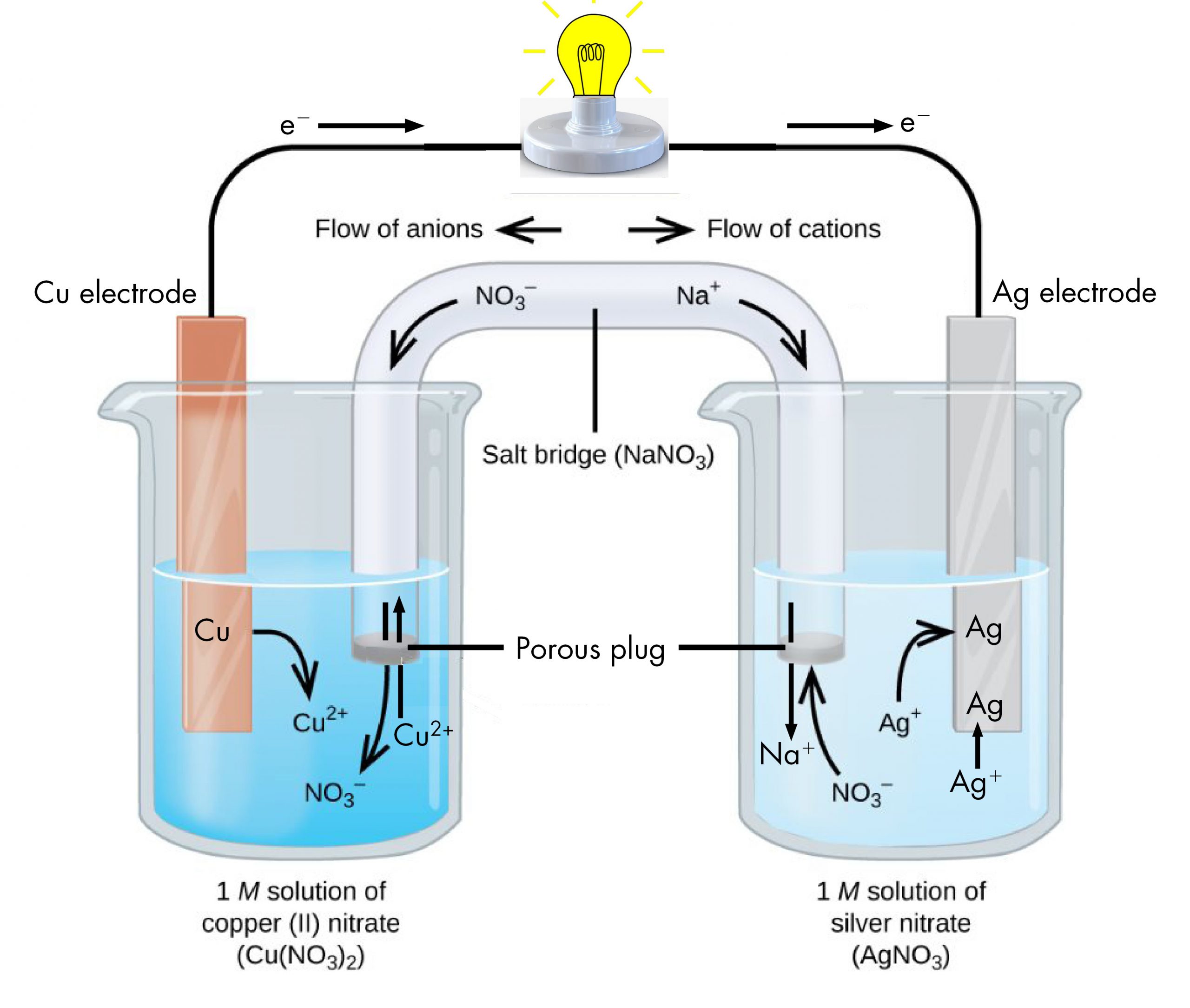
The following questions are about a silver-copper voltaic cell setup: a. Draw a labelled diagram to explain how a silver-copper voltaic cell works. Your diagram must show the electrodes, the salt bridge,

Calculate the concentration of silver ions in the cell constructed by using 0.1M concentration of Cu^2 + and Ag^+ ions. Cu and Ag metals are used as electrodes. The cell potential is